Talented ceramist required


We have now moved to the new lab so if you are planning on popping in or sending us anything, you'll need the new address which is:
Ambridge Ceramics
Premier House
Kiln Court...
College Road
Ripon
North Yorkshire
HG4 2BP
All the existing phone numbers etc. are now working as normal.
Thankyou for your patience during our move.
This year the focus was on Digital Dentistry and the growing use of CAD/CAM restorative solutions within implant dentistry.
We'd like to firstly thank everyone for the fantastic feedback and positive comments on the day, but also present a few of the key slides again here as they answer the most common questions that came to us after the presentation.
There is so much information entering the profession regarding the developments of Digital Dentistry and CAD/CAM but unfortunately this does seem to leave some confusion over some key benefits, and exactly why the move to Digital or CAD/CAM can be so beneficial when restoring implants.
Below are a few questions we received and we will try to provide as clear an answer as we can, based upon our years of experience with a very wide range of CAD/CAM system, services and partners out there.
Is CAD/CAM really more accurate?
This recent study again confirms that the fit of CAD/CAM structures (both Zr and Ti tested here) were significantly better than that of cast frameworks, the results of this study are below:-
The microgap between the Zirconia beam and the abutments was between 10-26 μm,
The microgap between the Titanium beam and abutments was between 6-18 μm,
The ‘misfit' of the Cast metal beam was between 181-301 μm.


We just want to make sure that all our clients get plenty of notice so we can ensure no patients are left without their smiles for Christmas.
You can also download the pdf to print and put up on your wall by clicking the link below.
https://dl.dropboxusercontent.com/u/3248981/Christmas%20Closing%202014.pdf
The Dental Laboratories Association are increasing their campaign of The British Bite Mark, a campaign that aims to raise public awareness of where their medical devices are made and as Ambridge Ceramics were one of the earliest members of the scheme we wanted to explain a little more about how we see The British Bite Mark benefitting the public and surgeons that choose to use a high quality lab.
The vast majority of the public are not aware of the different standards of traceability and skill required by each nation that produces dental restorations but in the UK the GDC and MHRA have gone to great lengths to protect patient safety by demanding high levels of traceability, compliance, professionalism and a commitment to quality of care to patients from their members.
Because all this regulation has been laid out by these bodies solely to protect patients, it seems a good idea to ensure your restorations are made by technicians that have to be registered with and answer to these bodies.
However there are many dental restorations that leave the UK and this protective framework to be made in countries which do not have the same standards or traceability requirements, often without the knowledge of the patient who will have the medical device fitted.
This is not a simply case of trying to claim that work from one country is good and that from another is bad, but simply asking why the work is being sent offshore? If it is for economical reasons then is the patient fully aware of the option to have the work made within the protective framework setup by a skilled, GDC registered technician and what they are getting for their money?
Is the patient benefitting financially from the cheaper work being made by a lab in a country that is not regulated by the GDC or MHRA? Or is the decision being made on their behalf and they are not being given the opportunity to make a choice between saving money or having the peace of mind knowing that their restorations were made by a skilled GDC registered professional?
The key to the whole issue is patient information and consent. If the patient is aware of all the information and still prefers to save money on their medical devices then that is fine, it's simply like the medical tourism which we have seen over the years.
However it's not maybe as obvious for that when many patients are unaware that their restorations were not made by a GDC registered technician within the protective system setup by the MHRA and GDC.
These people are unfortunately unwitting medical tourists who have not had the opportunity to be presented with the facts and weigh up the cost/benefits for themselves.
We live in a massive global economy and there is undoubtedly a benefit to using cheaper labour to keep production costs low, I think we are all aware that most of the technological devices etc. and many of the things we use every day are made this way?
But when we select medical devices and things we consume maybe we would make different choices if the fact were presented to us? The recent spate of health scares seems to indicate that some of the cost cutting in these areas has not been in the best interests of the consumers.
Although we don't agree with medical tourism (especially after seeing some of the shocking results that we have had to fix along with our skilled surgeons when some of these patients have come back to them after things have gone wrong) I can see why some people choose the apparent cost savings up front. However, I don't think it can ever be right that a patient becomes an accidental medical tourist because they are having restorations fitted that they are unaware have been made in an unregulated country.
At Ambridge Ceramics we have ploughed hundreds of thousand of pounds into new technology and technician training to continually improve the service we provide to our surgeons and patients, along with submitting to further voluntary independent inspection by a 3rd party assessor to ensure we meet the DAMAS standard ensuring we meet the highest possible levels of traceability and conformity on all our technician training and materials used.
Joining The British Bite Mark was just another logical step in our commitment at Ambridge Ceramics to providing our surgeons with the highest level of quality, traceability, service and support available in the UK, we passionately believe that the standards required to work as a GDC registered technician in UK labs are among the highest in the world and the work that comes out of our lab is also among the best seen anywhere in the world.
If you are a surgeon that uses a British Bite Mark laboratory then please don't waste the opportunity to promote this to your patients, it's a mark of the commitment to excellent quality and service you provide. It may also help to explain to the patient why your service could be slightly more expensive than the practice down the road. Are they offering the same exceptional levels of quality and care?
You are offering a superior level of service, quality and traceability, so why not make that clear to the patient? Be proud to stand out and be known for focussing on offering the very best quality and service you possibly can.
We have patient information booklets available that you can get by giving Sean a call on 01765 607347 or by contacting The Dental Laboratories Association directly by following this link
So when the campaign hits full swing and more patients start to ask "What's in my mouth?" you will be able to point to The British Bite Mark and demonstrate your commitment to offering restorations that are made in a lab, governed by the MHRA and produced by a GDC registered professional dental technician.
The Dental Laboratories Association have launched a dedicated British Bite Mark website that surgeons can use to find a laboratory but more importantly they have ensures that patients can find a surgeon that can supply them with these British Bite Mark restorations.
Please look at the site and ensure you are listed, this is something we have already started to do this for all clients of Ambridge Ceramics.



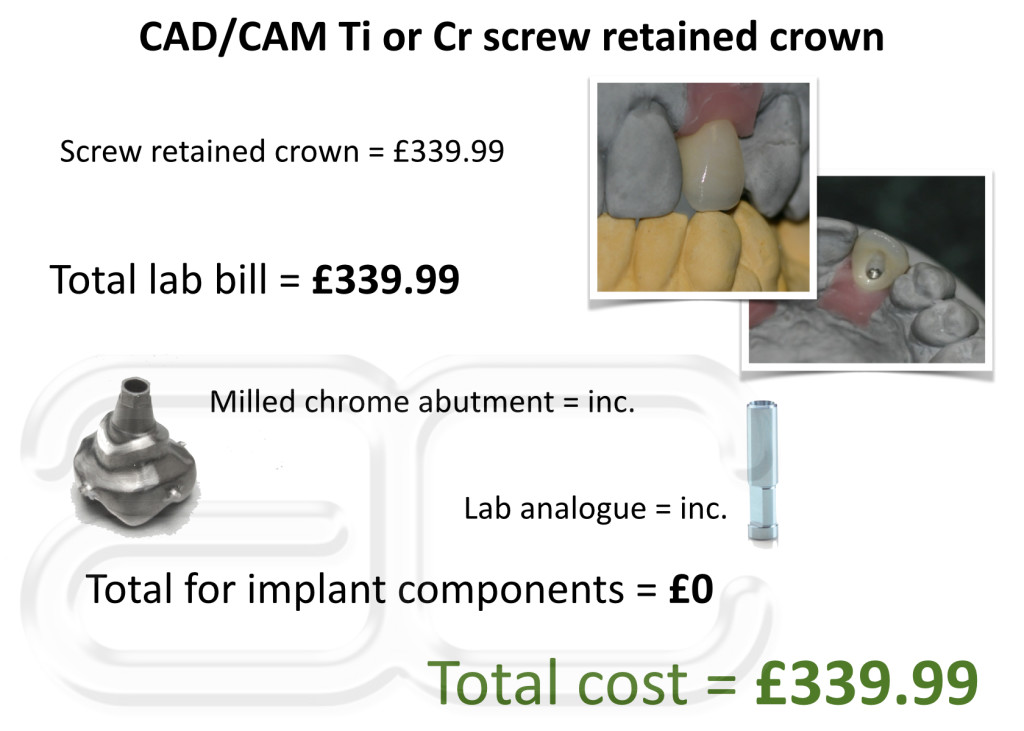


The titanium screw retained crowns will be officially available to the UK late 2014 but we will continue to offer this and many other unique solutions to our clients up until their official launch date.
For more information or to order your screw retained implant crowns you can either contact us by email
sean@www.ambridgeceramics.co.uk or Call Sean on 01765 607347.
The team were fortunate enough to be asked to contribute their knowledge of Digital Dentistry and CAD/CAM restorations to a challenging case that Dr Mark Willings carried out using the immediate loading technique with his team at Dental Excellence Harewood.
It's always very satisfying to see such great result when working with new implant systems and techniques.
We've been working with the Dental Wings system for many years now to create digital scans of our implant cases, and it's a fantastic system that just gets better year on year.
The case was planned and produced here in the UK, which is a great example of a British Bite Mark laboratory always pushing forward and using the latest technology for our surgeons and their patients.
You can read the full print article using the link below
https://www.dropbox.com/s/vm81oke26nnmicg/Gerald%20Crane%20case.pdf?dl=0